Patient With Myelofibrosis and Limited Treatment Options Due to Pancytopenia
Presentation and Diagnosis
M.M. is a 63-year-old male who was initially diagnosed with JAK2-negative primary myelofibrosis in 2006. He did not require therapy for many years; however, in 2019 he developed worsening cytopenias, weight loss, and splenomegaly. At that time, a repeat bone marrow biopsy was performed, which showed 3+ reticulin fibrosis, marked erythroid hyperplasia, and megakaryocytic hyperplasia without increased blasts, consistent with persistent primary myelofibrosis. Cytogenetics were diploid, and mutation analysis was positive for CALR. However, a full next-generation sequencing panel was not run. M.M. was categorized as DIPSS plus high risk based on the following clinical features of his disease:
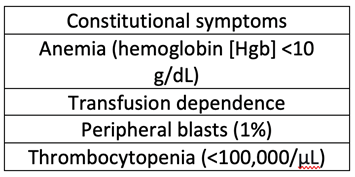
He was started on ruxolitinib, and after initiating this oral JAK inhibitor, he had improvement in quality of life, reduction in spleen size, and overall stability of disease. Due to his performance status and disease risk, M.M. was referred to our center for stem cell transplant consultation.
Interim Monitoring
At the time of referral for possible stem cell transplant, M.M. had a white blood cell (WBC) count of 4,000/μL with 2% blasts, Hgb 8.6 g/dL, and platelets of 50,000/μL. He was receiving 1-2 units of packed red blood cells every month for supportive care, which was keeping his Hgb >7 g/dL and helped with symptoms related to his anemia. During the search for a donor, M.M. continued his current ruxolitinib dose, which provided benefit. Unfortunately, his WBC and platelets started to drop and did not recover despite a dose reduction in the ruxolitinib (WBC 2,300/μL, platelets 26,000/μL).
At this point, identifying an appropriate donor for M.M. proved to be very difficult, and given the need for a change in therapy, he opted for enrollment onto a clinical trial. He was required to have a washout from ruxolitinib prior to starting on the clinical trial, and aside from rebound symptoms lasting a few days, he tolerated this well.
Clinical Trial Enrollment
M.M. enrolled onto a blinded phase 3 randomized clinical trial with momelotinib/placebo daily plus danazol/placebo twice daily. He tolerated this well for 2 months, yet without substantial improvement in counts. Prior to cycle 3, clinical trial medications/placebo were held secondary to the development of grade 4 neutropenia (absolute neutrophil count 400). Again, despite therapy hold, M.M.'s counts did not recover, and he was taken off the trial.
Stem Cell Transplant
M.M. was evaluated yet again for stem cell transplant, and after many discussions with a variety of experts, he decided to proceed with transplant with his grandson as the identified haplo-identical donor. M.M. is currently admitted for transplant awaiting engraftment.
Considerations
Patients who have pancytopenia as a feature of their myelofibrosis are very challenging to manage. JAK inhibitors require certain levels of dose adjustments for cytopenias, and some patients reach a point when they cannot safely remain on JAK inhibitor therapy. There are currently no approved therapies known to reverse bone marrow fibrosis or improve pancytopenia. Patients in this difficult situation should be considered for stem cell transplant or clinical trial enrollment. Supportive care with transfusions and antimicrobial prophylaxis may also be necessary.
