A frank discussion of the logistical challenges associated with applying guideline recommendations to patients with NSCLC in the United States. The paper reviews commonly used tissue- and blood-based biomarker testing techniques as well as the challenge of interpreting results and using results to guide treatment decisions.
This narrative review published in Oncology explores various aspects of decision-making in oncology, including criteria for making decisions and shared decision-making. The authors note some of the limitations in current models of shared decision-making and highlight the influence of contextual factors in decision-making.
This paper reviews the rationale for molecular testing in patients at initial diagnosis of advanced NSCLC and progression on targeted therapy and proposes real world strategies to optimize the molecular testing in newly diagnosed patients with advanced NSCLC.
These updated guidelines from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology offer 18 new recommendations, including ROS1 testing for all adenocarcinoma patients.
This study evaluated the efficacy of NGS by comparison to ddPCR assay and Sanger sequencing using the Iontorrent personal genome machine (PGM) detect somatic mutations. NGS provided rich genetic information and reduced the cost and time of sequencing, with high output and high resolution.
Although the transition to molecularly defined patient subgroups in advanced non–small-cell lung cancer often leads to dramatic and prolonged responses to an inhibitor of an identified oncogenic mutation, acquired resistance eventually ensues. The optimal approach to management in that setting remains the subject of ongoing research.
~25,000 patients newly diagnosed wth NSCLC each year have a KRAS G12C mutation. This fact sheet from Amgen succinctly reviews biomarker testing modalities to identify genomic alterations as the foundatin for treatment.
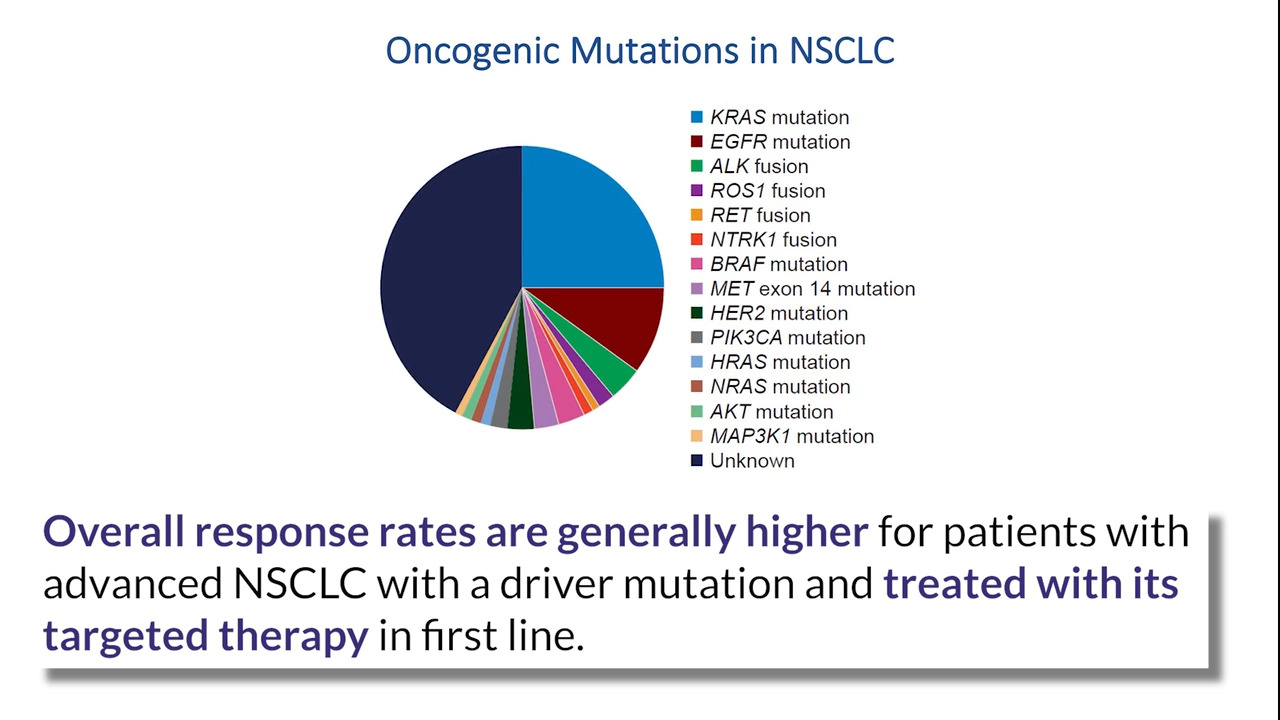
KRAS G12C is a prevalent driver mutation in lung adenocarcinomas. This informational slide deck from Amgen provides an overview of KRAS G12C as a molecular target in NSCLC, including the heterogeneity of KRAS g12C-mutated tumors, targeted therapies with potential to lock the KRAS G12C mutant protein in the inactive state, and molecular testing guidelines to assess treatment options for patietns with advanced NSCLC.
The most recent National Comprehensive Cancer Network clinical practice guidelines for managing patients with NSCLC includes recommendations concerning molecular and biomarker testing and analysis.