Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer
Last Updated: Wednesday, September 22, 2021
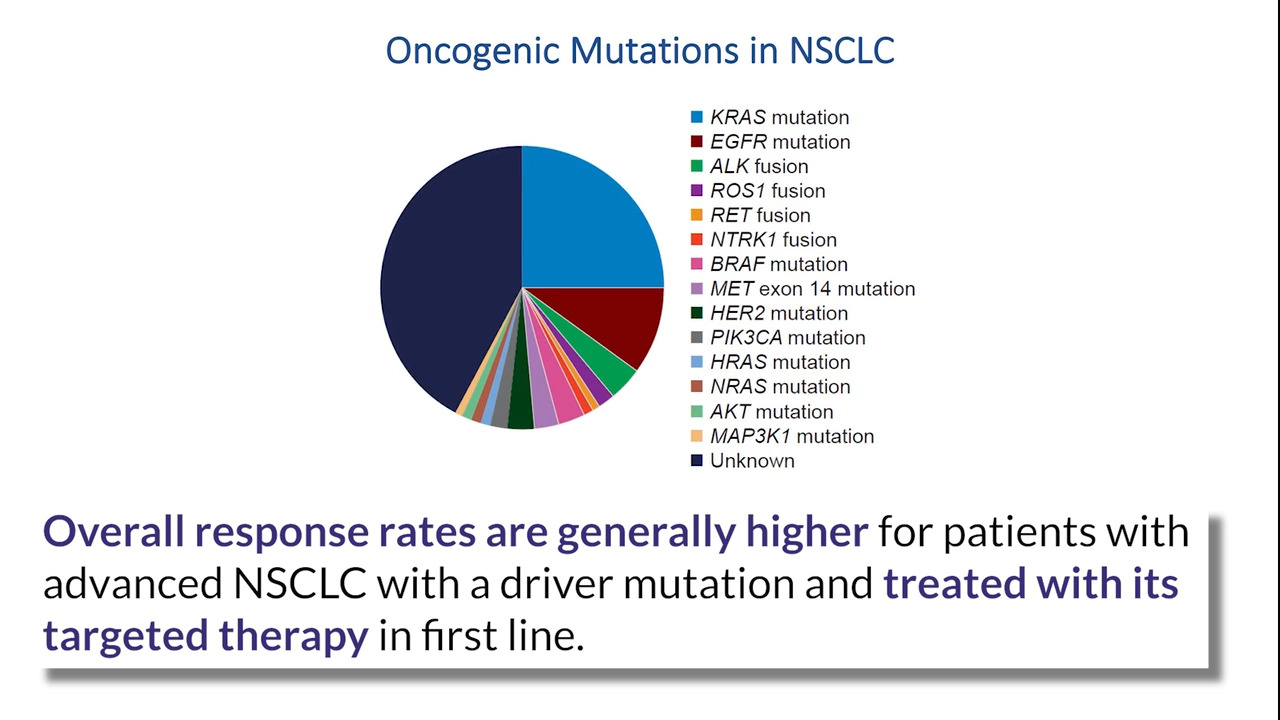
In view of the novel and impressive technological advances made in the past few years, the growing clinical application of plasma-based, next-generation sequencing, and the recent Food and Drug and Administration approval in the United States of two different assays for circulating tumor DNA analysis, IASLC revisited the role of liquid biopsy in therapeutic decision-making in this 2021 update.
Advertisement
News & Literature Highlights