Defining Comprehensive Biomarker-Related Testing and Treatment Practices for Advanced Non-Small-Cell Lung Cancer: Results of a Survey of U.S. Oncologists
Last Updated: Tuesday, February 22, 2022
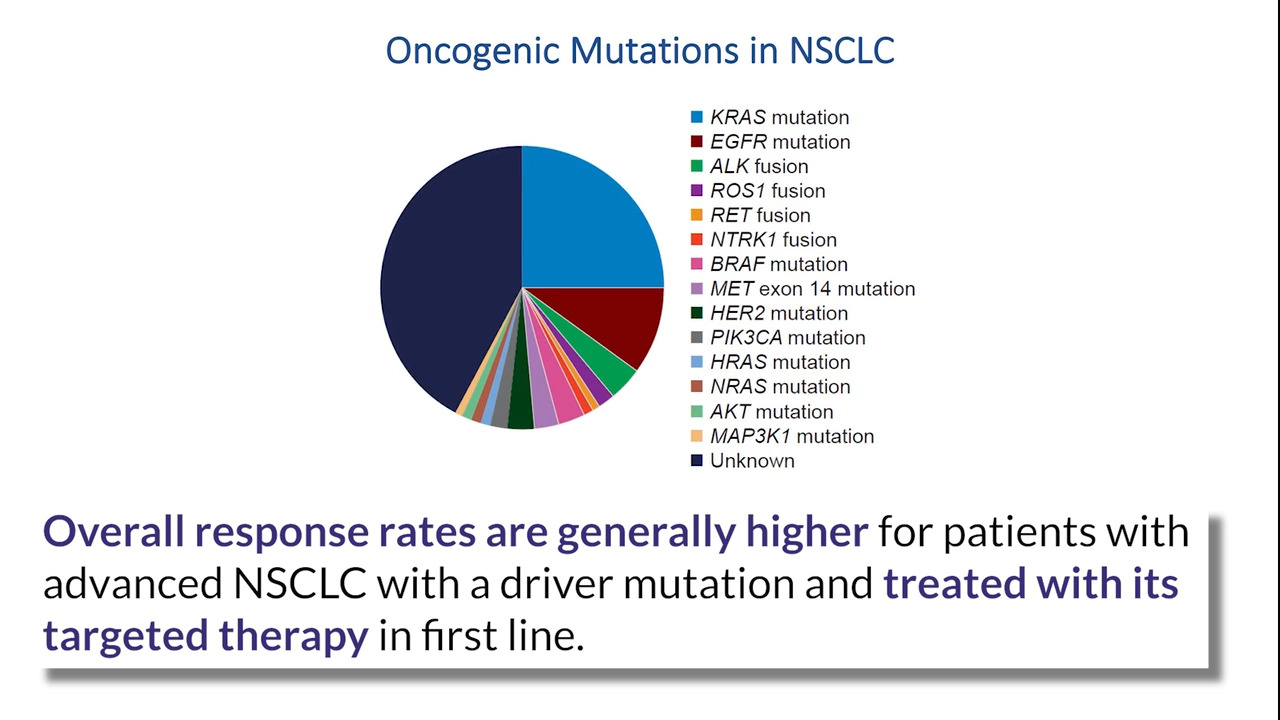
An ASCO taskforce comprised of representatives of oncology clinicians, the American Cancer Society National Lung Cancer Roundtable (NLCRT), LUNGevity, the GO2 Foundation for Lung Cancer, and the ROS1ders sought to: characterize U.S. oncologists’ biomarker ordering and treatment practices for advanced non-small-cell lung cancer (NSCLC); ascertain barriers to biomarker testing; and understand the impact of delays on treatment decisions. Respondents reported high biomarker testing rates in patients with NSCLC. Treatment decisions were impacted by test turnaround time and associated with practice setting and physician specialization and experience.
Advertisement
News & Literature Highlights