Blood First Assay Screening Trial (BFAST) in Treatment-Naive Advanced or Metastatic Non–Small Cell Lung Cancer: Initial Results of the Phase 2 ALK-Positive Cohort
Last Updated: Monday, August 16, 2021
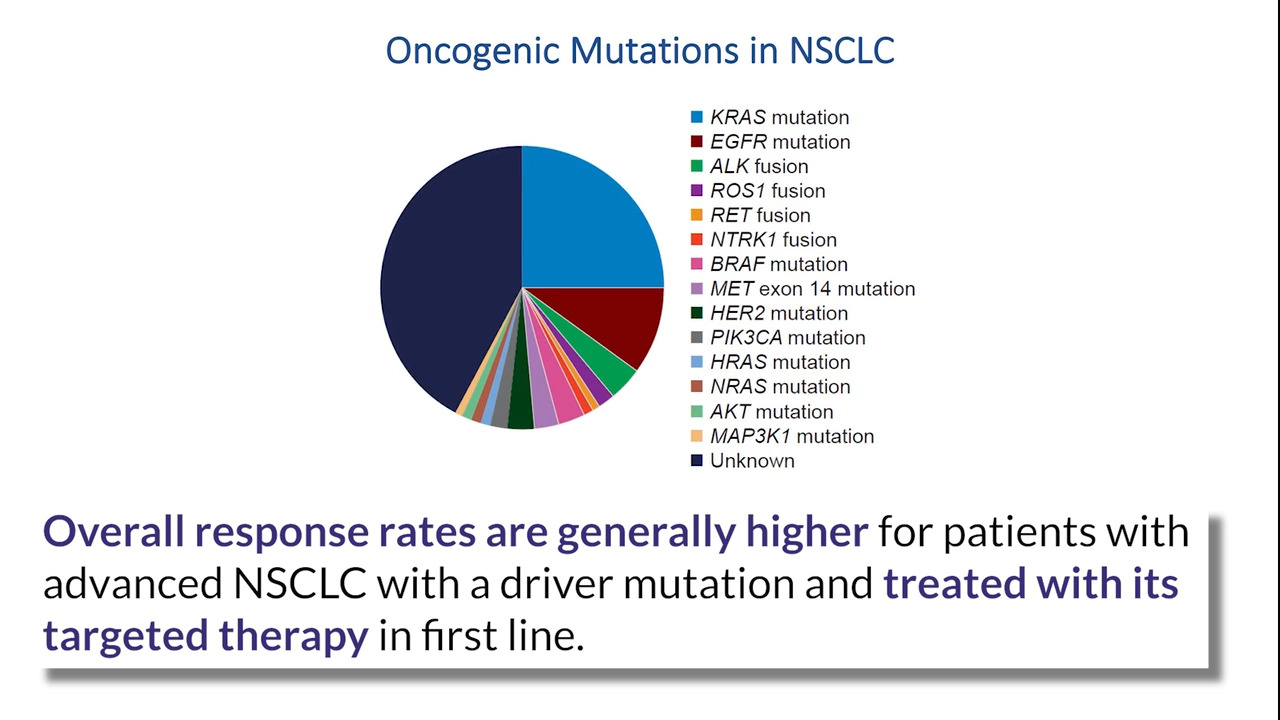
The Blood First Assay Screening Trial (BFAST) is an ongoing open-label, multi-cohort study, prospectively evaluating the relationship between blood-based next-generation sequencing (NGS) detection of actionable genetic alterations and activity of targeted therapies/immunotherapy in metastatic NSCLC. In this article, the authors present data demonstrating the clinical application of blood-based NGS as a method to inform clinical decision-making in ALK-positive NSCLC.
Advertisement
News & Literature Highlights